Dyness Knowledge | Energy storage terminology: Energy density, self-discharge rate & cell consistency
Post by the Dyness Technical Team | 25/01/2024 6-minute read Energy density, self-discharge rate and cell consistency are key to achieving efficient storage and utilisation of stored electrical energy within a battery system, but what do these three terms actually mean, and how do they impact performance and lifespan? Here, members of the Dyness team explain the parameters and why they are so important in battery design and manufacture.Cells, packs and systems
Before we get into the details, we should define the building blocks of a battery storage system as we’ve referred to them in this article.
Battery cell: when we refer to a cell, we’re talking about the fundamental element of a battery that stores and releases energy by conversions between chemical energy and electrical energy. A cell consists of two electrodes, one positive (a cathode) and one negative (an anode), an electrolyte to transfers ions (charged particles) between the electrodes, and a diaphragm. The diaphragm is a layer that separates the electrodes and has microscopic holes to allow the passage of the ions.
Battery pack: this is made up of a number of battery cells.
Battery system: this is made up of a number of battery packs along with other system elements.
Energy Density
Energy density refers to the amount of energy stored per unit of space or mass of matter. You may see it expressed as gravimetric energy density and volumetric energy density. The gravimetric energy density indicates how much energy per kilogram or gram can be stored, while its volumetric energy density shows how much energy per litre can be stored. It follows that the greater the energy density of the battery, the more electricity can be stored per unit volume or weight. There are two different concepts of battery energy density, one refers to the energy density of a single cell, and the other is the energy density of a battery system as a whole. Let’s consider the whole system:Improving system energy density
The energy density of the battery system relates to the weight or volume of the entire system which includes a number of elements such as the battery management system, thermal management system and high and low-voltage circuits in addition to its storage elements. Its energy density can be improved by:- Increasing the energy density of the battery packs.
- Improving the grouping efficiency of the battery packs. This can be achieved by:
-
- Optimising the layout to make the battery pack more compact.
- Shape and topology optimisation: This is way of improving the distribution and layout of materials within a given space by using simulations and mathematical calculations. It is an effective way of reducing the weight of a component whilst maintaining its rigidity and structural integrity.
- Choosing low-density materials, for example, materials used for battery pack covers and the lower box of the battery packs have changed from sheet metal to composite materials, reducing the weight by around 35% and 40% respectively.
- Optimising overall system design and the heat dissipation systems by improving the layout of each element.
Self-discharge Rate
Even if there is no external load, the battery will discharge due to the internal chemical reactions within the battery cells when it is not in use. This is also expressed as the charge retention capacity. The self-discharge rate is affected by factors such as the manufacturing process, raw materials (caused by the impurities in the metals and electrolytes) and storage.SOC: It’s important to understand one more term when we talk about the issues associated with self-discharge, i.e. the state of charge of a battery cell, abbreviated to SOC. SOC is the capacity of a battery cell that is currently available. It’s expressed as a percentage of its rated capacity and so ranges between 0 and 100%. An SOC of 0% would mean that a cell is completely discharged and an SOC of 100% indicates that it is fully charged.
There are a number of issues associated with self-discharge
- Self-discharge is accompanied by an increase in battery internal resistance, resulting in a decrease in battery load capacity.
- Over-discharge may lead to a loss of capacity that cannot be reversed.
- Self-discharge caused by metal impurities can cause the apertures in the diaphragm to be blocked. These can even pierce the diaphragm resulting a local short circuit which can endanger the safety of the battery.
- Self-discharge leads to an increase in the SOC difference between battery cells, leading to a decrease in the capacity of the battery pack.
- Large differences in SOC can lead to overcharge and over-discharge of the battery.
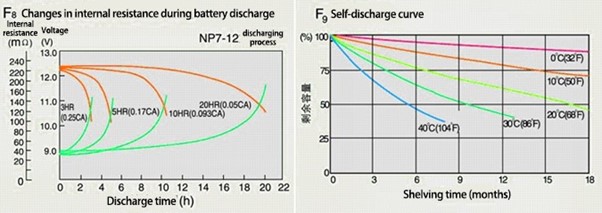
Reducing the self-discharge rate
Lithium-ion batteries are known for having a lower self-discharge rate than other re-chargeable batteries but correct storage and avoiding long-term non-use are still important. These batteries should always be stored in a dry, cool and well-ventilated area as per the manufacturer’s recommendations. Good design is also key. Reducing the surface area of metal materials reduces the self-discharge rate.Cell Consistency
Inconsistencies between cells impacts both performance and the lifespan of a battery pack. When we talk about cell consistency, we include cell capacity and resistance, the electrical characteristics of the electrodes, electrical connections, temperature characteristics and rates of decay.- Capacity loss: Lithium battery packs are made up of a number of single cells and the overall capacity of a pack conforms to the theory of wooden barrels, where the capacity of a barrel is determined by the shortest wooden bar. Similarly, no matter how good the greatest performing cell is, the capacity of the worst cell determines the capacity of the entire battery pack.
- Reduction in lifespan: Lower-capacity cells are likely to become fully charged and discharged every time the battery is used, causing them to reach their end of life at an earlier stage than others. When one battery cell gets to its end of life, the whole group of battery cells will also reach end of life.
- Deterioration due to internal resistance: With differing internal resistances and the same current flow, cells with greater internal resistances will generate more heat relative to others. If the temperature of the battery is too high, the internal resistance will further increase. This feedback of increasing internal resistance and temperature rise will accelerate deterioration.
Overcoming issues caused by cell inconsistency
There are a number of ways of mitigating cell inconsistency to improve battery performance and lifespan.- Screening: Screening and sorting are imperative. Battery cells from different batches should not be used together - even cells within the same manufacturing batch need to be screened to ensure that those with relatively similar parameters are placed within the same battery pack.
- Thermal management: For batteries with inconsistencies in internal resistance, the heat that’s generated will be distributed unevenly within the battery pack. The addition of a thermal management system enables the temperature difference of the entire lithium battery pack to be adjusted keeping it within a narrower range. The battery cells that have higher resistance will still generate more heat, but with thermal management, there will be no obvious difference in the level of degradation.
- Battery balance management, battery management system & BMS design balance: By regulating temperature, reducing vibrations and preventing ingress from water, dust and other pollutants, and by adjusting or replacing battery cells that go beyond defined limits in time, these systems and functions optimise the operational environment of the lithium battery pack.